Cathodic Protection Network
International

The specifications for the cathodic protection of the VRESAP pipeline include a compliance to DIN 50918. Permanent reference electrodes are specified as complying to these requirements but the design of these is similar to the 'isopotential cell' described in cathodic Protection Network technology and will not function as required by DIN 50918.
The specification is defined with a drawing of the arangement of pieces of metal, measuring instruments, containers and liquids. This always carried out in a laboratory and it should be noted that the arrangement is in a closed circuit condition. Pipeline field conditions have been described as 'open circuit measurements' in which a probe is placed in the electrolyte that is subject to infinitely variable electric flux.
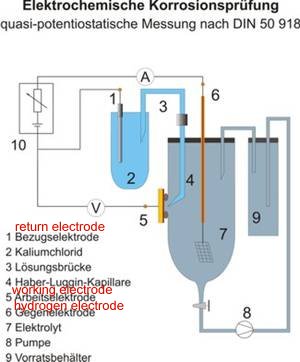
The specifications for this contract include a drawing that is not compliant with DIN 50918 and describes this as the IR Free Reference Electrode.

The data gathered from this monitoring system is incapable of defining the corrosion status of any pipeline.
The steel coupon reacts with the earth and corrosion takes place at micro-anodes that release charges to micro-cathodes on the same surface. The resulting corrosion current cannot be measured at the anodes of this system for two reasons.
1. It is impossible to locate and individual anode.
2. Placing any electrode in the active electrolyte at any anode disturbs the measurement.
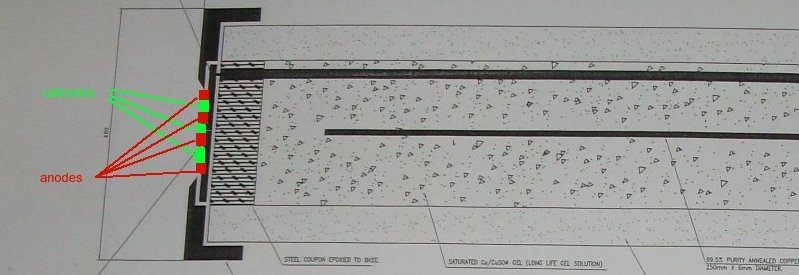
It can be seen that the laboratory arrangement separates the anode and cathode of the subject corrosion cell but the specified 'IR free reference electrode'has anodes and cathodes on the same surface.

The term IR drop is used in science to define the zone of potentials between the active electrode (sometimes called the working electrode) and the return electrode (sometimes called the counter electrode). Because of the volume of electrolyte, the concentration of charges decreases as the diffuse from the reaction and the potential of the electrolyte forms zones that have been likened to the rings of an onion and called shells of resistance.
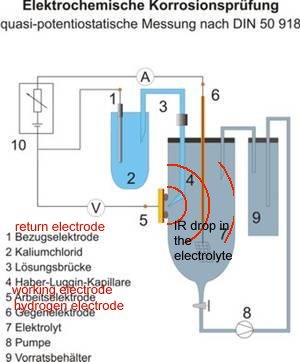
It is to overcome this problem of the IR drop (that is not a measurable voltage) that the Lugin Capillary was devised to place a conductive but non-reactive electrical path into the zone of the electrolyte that is subject to the complete EMF of the reaction.
The measurement resulting from the specified IR free reference electrode and 'IR free' terminal card will contain errors resulting from variable electrical flux in the ground itself.
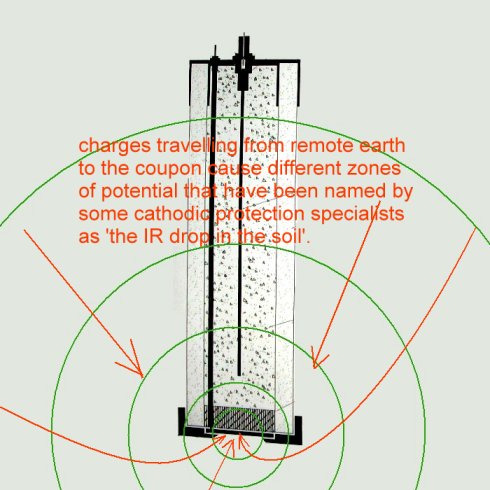
This includes the influence of the CP current going onto the coupon to return to the TR negative connection. Switching off the impressed current system removes some of the errors but leaves the pipeline in a abnormal condition. I order to ascertain the normal corrosion status of the pipeline we must include the effects of all electrical influences including sacrificial and impressed current systems belonging to all parties.
It is for this reason that the Alexander Cell was designed 30 years ago and now incorporates a method of measuring the potential of the electrolyte at the anodic interface in the same way as the arrangement in the laboratory.

The newly patented model has been bench and field tested at the Cathodic Protection Network International Trainign and Research Centre, Guararema, Brazil.

Return to main report

